Why are Valence Electrons Important? Learn About the Role & Importance of Valence Electrons
Studying the Atom
To begin with, we have to learn a little about the structure of an atom. An atom is made up of protons, neutrons, and electrons. It is a basic unit of matter, consisting of a nucleus and electrons (negatively charged particles) that are found in orbits around it. The nucleus is what contains the protons (positively charged particles) and neutrons (uncharged particles). The electrons rotate around the nucleus, in the same fashion as the sun revolves around the planets.
What Are Valence Electrons Exactly?
Valence electrons are the electrons that reside in the outermost electron shell of an atom in the highest energy level. They are important to an atom because the fewer valence electrons that the atom holds, the less stable it becomes.
What Functions Do They Perform?
Valence electrons are the most exposed of all the electrons, essentially acting as a protective barrier for the rest of the atom. Because they are in the highest energy level, they are generally the most involved in chemical reactions since they are the easiest to transfer. The function of valence electrons is to transfer between the atom, gaining or losing electrons in the process, in an effort to bring about stability to the atom.
Valence electrons are such an essential part of the atom’s stability that an atom will be reactive or inert depending solely on how many valence electrons it has. For the most part, eight valence electrons are necessary for an atom to reach a state of stability.
Why Are They Important?
As mentioned earlier, the number of valence electrons the atom holds the more stable it is. In addition to that, the amount of valence electrons in the outer shell determines how atoms interact with one another. Based upon the information from these two statements, it’s easy to see just how important valence electrons are to an atom.
To understand how an atom reaches stability it’s vital to know the function and importance of valence electrons. Without these electrons, the atom simply could not exist. It provides a variety of functions that enable it to create a foundation for chemical bonds.
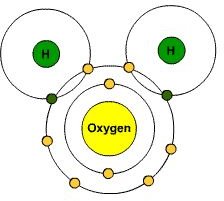
We know that water consists of two hydrogen elements and one oxygen element. We must drink water to survive. It’s necessary to use water in our everyday lives. It’s essential to our living. But with all that being said, I highly doubt many people understand the complex procedure that had to take place to make this water. For water to achieve stability and form a covalent bond, oxygen has to gain two atoms, while hydrogen has to gain or lose one atom. The two hydrogen atoms and the oxygen share the electrons, which, in turn, form a water molecule.
Water is one of many interesting examples of how valence electrons are at work in our world today. Take a look around you. Our earth is simply filled with endless beautifully complex creations, much to the thanks of the work of valence electrons.
The Water Molecule
Resources
https://www.reference.com/browse/valence+electrons
https://dl.clackamas.cc.or.us/ch104-06/valence_electrons.htm