Prove Atoms Exist: Trye These 3 Science Experiments in Your Class to Prove that the Invisible Atom Exists
The World of the Unseen
Atoms are the smallest particle of matter – invisible – but they are what bonds together to make a molecule. From this small building block elements are built which in turn make molecules, then macromolecules are built which continue with building organisms, which continue to make planets, and from the tiniest particles known as atoms the Universe is built.
Activity: One Lego set constructed and 3 to 4 separate Legos. While a Lego block is MUCH larger than an atom, it can be used to demonstrate how when joined it can make something.
Experiment 1: Water is Full of Holes!
Water molecules create invisible spaces in water. Let’s prove it! I have 1 cup of water and 1 cup of rubbing alcohol. 1 + 1 = 2 ~ right? Let’s see….
Materials:
- 1 cup water in a 4 cup clear glass container
- 1 cup rubbing alcohol
Steps:
*Note:measurements must be exact.
1. Have the 1 cup of water in a clear glass container.
2. Pour 1 cup of rubbing alcohol into the water.
What are the results? Less than 2 cups! Why? The molecules in the rubbing alcohol have filled the holes in the water.
Experiment 2: If it’s Invisible Can it Still Move?
Materials:
- Glass jar
- Water
- Food coloring
*Note:Jar must be somewhere it will not be disturbed for a couple of hours.
1. Fill jar about half way with water.
2. Add several drops of food coloring.
At first the food coloring will move to the bottom of the jar, but after several hours, the water is all one color. WHY? The invisible molecules (proven in experiment 1) are always moving and will ‘stir’ the water.
Experiment 3: Does a Hot Molecule Dance Faster Than a Cold One?
Materials:
- 2 glass jars
- Water
- Food coloring
1. Place 1 cup cold water in the first jar and 1 cup HOT water in the second.
2. Add 2 drops of food coloring in each jar.
3. Leave jars undisturbed.
Results: Hot molecules dance faster ~ the jar of hot water will quickly become one color where the cold will take most of a day.
Model 1: Make a Water Molecule
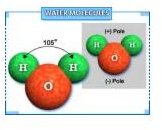
A water molecule is the connection of two Hydrogen atoms and one oxygen atom.This is called h2o and looks like mouse ears.
Materials:
- Large white marshmallow
- Bags of small colored marshmallows
- Toothpicks
Steps:
1. Have the students select two of the colored marshmallows. The large marshmallow will be oxygen atom which is 16x larger.
2. The students will connect the two smaller marshmallows to the larger one to make mouse ears.
Model 2: Ice Crystals
*Note:This is a continuation to the above project.
Frozen water molecules bonded into 6-sided ice crystals.
Materials:
- Large marshmallows
- Small colored marshmallows
- Toothpicks
Steps:
1. From the original water molecule have the students add an oxygen atom.
2. On the new oxygen atom the students will place a hydrogen atom (making mouse ears).
3. Continue until they have constructed a 16-sided ice crystal.
Model 3: Salt Molecules
A salt molecule is made up of one sodium atom and one chlorine atom. Where ice crystals are 16 sided, salt molecules are square.
Materials:
- Large marshmallows
- Small colored marshmallows
- Toothpicks
Steps:
1. Have students connect the two small marshmallows to a large one in at 90 degrees.
2. Then from the each small one they connect a large one – still 90 degrees with a box being the end result.